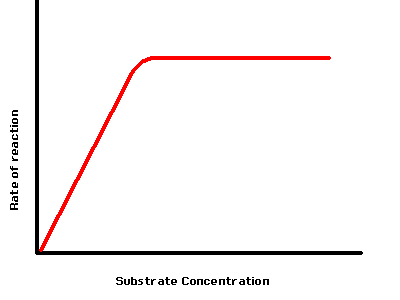
The Effect of Concentration of Substrate on the Rate of an Enzyme Controlled Reaction
If you increase the concentration of the substrate (the chemicals involved) in an enzyme controlled you will speed up the chemical reaction. The reason for this is as follows: The substrate and the enzyme need to come into contact with each other for a reaction to proceed. When the two meet they join for a brief moment while the reaction is catalysed, before separating. If there are very few substrate particles in the solution, it will take time for them to bump into an enzyme molecule and therefore the reaction will be slow.
Increasing the substrate concentration means the chance of the collision between a substrate molecule and the enzyme is increased also. A little like packing more people into a room. The more people there are the more people you would bump into.
This does not increase indefinitely though. There comes a point when the enzyme is working as fast as it can. As soon as one substrate molecule is converted into the products, another one comes along and takes its place. The time taken for the enzyme to catalyse the reaction when they come into contact with each other limits how fast it can go.